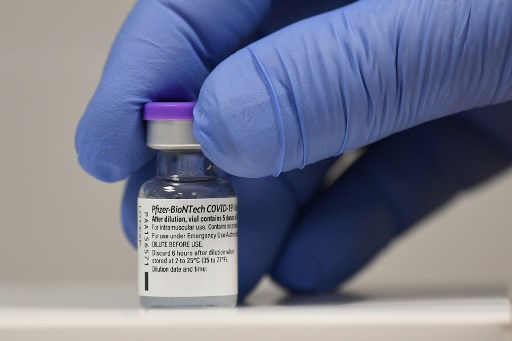
THE HAGUE, Netherlands (AFP) — The Pfizer/BioNTech coronavirus jab has no link to reported post-vaccination deaths and no new side effects, the EU’s medicines regulator said Friday based on the first data from the vaccine’s rollout.
The European Medicines Agency said it had looked at the deaths, including a number in the elderly, and “concluded that the data did not show a link to vaccination with Comirnaty (the vaccine) and the cases do not raise a safety concern.”
In its first safety update since the EU started its vaccination campaign in December, the Amsterdam-based EMA said that data “is consistent with the known safety profile of the vaccine, and no new side effects were identified.”
Reports of occasional severe allergic reactions did not go beyond what had already been found about this “known side effect”, it added.
“The benefits of Comirnaty in preventing Covid-19 continue to outweigh any risks, and there are no recommended changes regarding the use of the vaccine,” the EMA said.
The EU watchdog has so far approved two vaccines, by Pfizer/BioNTech and Moderna.
It is set to give its verdict on a third, by AstraZeneca, later Friday.
A number of countries, including Norway, Denmark, Finland, Iceland and Sweden, have reported deaths of people who had been given the Pfizer-BioNTech jab but no direct links to the vaccine have been established.
Norway in particular registered 33 deaths among elderly people who had received their first dose.
Oslo said earlier this month it had not established a link but recommended doctors consider the overall health of the most frail before giving them the jab.
© Agence France-Presse