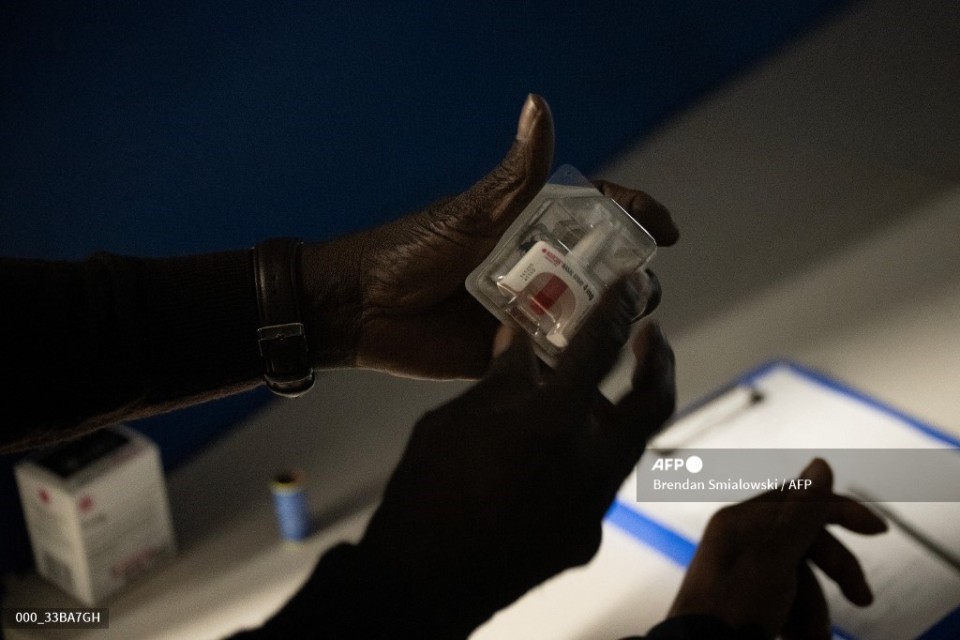
The nasal spray, which has been authorized in the United States since 2015, has become an indispensable tool in the fight against the opioid epidemic gripping the country. (Photo by Brendan Smialowski / AFP)
Washington, United States | AFP | Wednesday 3/29/2023
The US drug regulator announced Wednesday that Narcan, the treatment which reverses overdoses, can be distributed without prescription, a move that comes as the country battles an acute opioid addiction crisis.
The Federal Drug Administration (FDA) said that four milligram nasal spray doses of naloxone hydrochloride, the generic name for Narcan, can be sold over the counter almost anywhere, from pharmacies to supermarkets to gas stations.
The FDA said they moved to address to mounting toll from opioid use, especially the synthetic and relatively easily made fentanyl, which has in the past five years increasingly replaced heroin and prescription pain killers as the main drug used by people addicted to opioids.
In the 12 months to October 2022, the US recorded 101,750 overdose deaths, mostly from fentanyl, the FDA said in a statement.
“The agency has used its regulatory authority to facilitate greater access to naloxone by encouraging the development of and approving an over-the-counter naloxone product to address the dire public health need,” said FDA Commissioner Robert Califf.
Narcan, by far the most commonly used version of naloxone, can quickly reverse the effects of an overdose.
Narcan will “bring someone back from basically the dead,” Johnny Bailey, who has recovered from an opioid addiction and who trains people to administer the drug, explained in early March in Washington.
Previously administered mainly by injection, Narcan, produced by Emergent BioSolutions, was approved in its nasal spray form as a prescription drug in 2015.
With the toll from overdoses mounting each year, the FDA accelerated its review of Narcan for over-the-counter sales early this year.
The FDA said other producers of naloxone spray can gain approval on a case-by-case basis.
“Naloxone is a critical tool in addressing opioid overdoses,” said FDA drug evaluation chief Patrizia Cavazzoni.
© Agence France-Presse